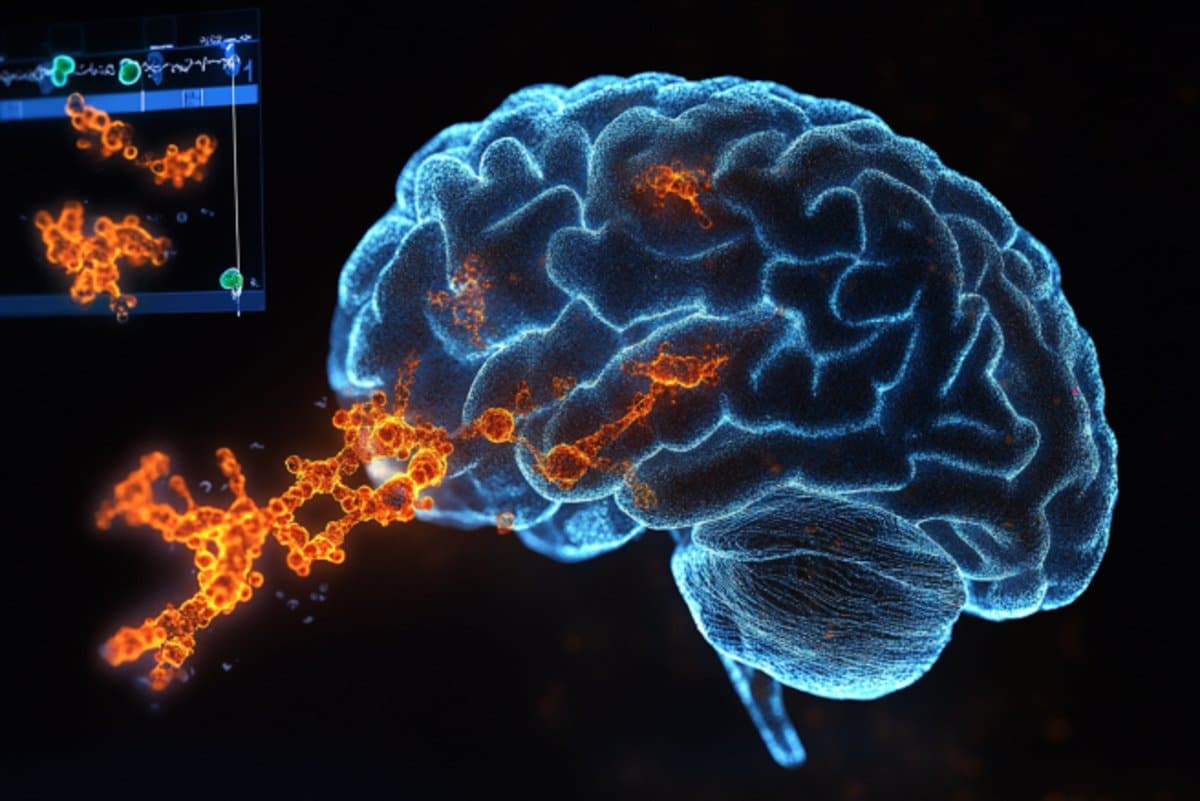
Recent advancements in neuroscience have led to the development of a groundbreaking technique known as EPSILON, which stands for Enhanced Protein Structural Imaging and Localization in Neurites. This innovative method offers researchers an unparalleled opportunity to explore the intricate world of proteins that play a crucial role in memory formation. By employing advanced imaging techniques, EPSILON allows scientists to visualize protein interactions and distributions within neurons, revealing insights that were previously obscured by limitations in traditional imaging methods. This level of detail not only enhances our understanding of the molecular architecture of memory but also paves the way for potential therapeutic targets in neurodegenerative diseases and cognitive disorders.
One of the most significant aspects of EPSILON is its ability to provide high-resolution images of proteins in their native cellular environments. Utilizing a combination of advanced fluorescent tagging and super-resolution microscopy, researchers can now observe how proteins involved in synaptic plasticity dynamically interact during memory formation. Synaptic plasticity is a fundamental process by which neurons strengthen or weaken their connections in response to activity, a phenomenon that underlies learning and memory. By mapping these protein interactions in real-time, EPSILON empowers scientists to dissect the molecular mechanisms that contribute to memory consolidation and retrieval, offering a clearer picture of how memories are formed and stored within the brain.
The implications of EPSILON extend beyond basic research into memory formation; this technique holds promise for understanding the molecular underpinnings of various neurological disorders. Conditions such as Alzheimer’s disease, schizophrenia, and autism spectrum disorders have been linked to dysregulation of protein interactions and synaptic function. By harnessing the capabilities of EPSILON, researchers can identify specific protein anomalies associated with these conditions, potentially leading to the development of targeted therapies aimed at restoring normal protein function and synaptic health. Furthermore, this technique may enable the identification of biomarkers that could be used for early diagnosis or monitoring the progression of neurodegenerative diseases, significantly impacting patient care and treatment outcomes.
In conclusion, the introduction of EPSILON marks a significant milestone in neuroscience, offering a powerful tool for mapping the proteins involved in memory formation with unprecedented detail. As researchers continue to unravel the complexities of the brain's molecular architecture, this technique not only enhances our understanding of fundamental cognitive processes but also opens new avenues for addressing the challenges posed by neurological disorders. The potential applications of EPSILON are vast, suggesting that as we deepen our understanding of memory at the molecular level, we may also uncover new pathways for therapeutic intervention, ultimately improving the quality of life for individuals affected by cognitive impairments.
How Memories Take Shape at the Synapse Level - Neuroscience News